Ketamine Regulations and Trends in Late 2025: A Growing Option for Mental Health Treatment

As of December 2025, ketamine stands out as the most accessible legal psychedelic for therapeutic use in the United States. Unlike emerging psychedelics like psilocybin (limited to supervised programs in a few states), ketamine—particularly off-label IV infusions and the FDA-approved esketamine nasal spray (Spravato)—is widely available through clinics and medical providers. This positions it as a bridge between traditional pharmaceuticals and the psychedelic renaissance, with rapid growth in clinics and increasing integration with psychotherapy.
Key Regulatory Status
- Federal Level: Ketamine is a DEA Schedule III controlled substance, indicating accepted medical use with moderate abuse potential (safer federally than cannabis, which remains Schedule I despite recent rescheduling efforts). IV ketamine for depression, anxiety, PTSD, and chronic pain is off-label but legal when administered by licensed providers. Esketamine (Spravato) received expanded FDA approval in January 2025 as the first monotherapy for treatment-resistant depression (TRD), allowing use without combining with oral antidepressants.
- Spravato Specifics: Administered only in certified healthcare settings under the REMS program—patients must be monitored for at least 2 hours post-dose due to risks of sedation and dissociation. No take-home use.
- Telehealth and At-Home Options: DEA telemedicine flexibilities for prescribing controlled substances (including ketamine lozenges/troches) are extended through December 31, 2025, allowing remote prescribing without in-person exams in many cases. This has fueled at-home models, but scrutiny is rising—providers must comply with record-keeping and avoid diversion risks.
- State Variations: Regulations are patchwork. Most states allow off-label clinic use, but some impose restrictions on ownership (e.g., corporate practice of medicine laws requiring physician ownership), provider types, or telehealth. No state bans therapeutic ketamine outright, but clinics need DEA registration, secure storage, and inventory tracking.
Clinic Trends and Growth
The ketamine clinic market is booming, projected to grow at 11-15% CAGR through 2032, driven by demand for treatment-resistant conditions. Models include:
- In-clinic IV infusions (most common, supervised).
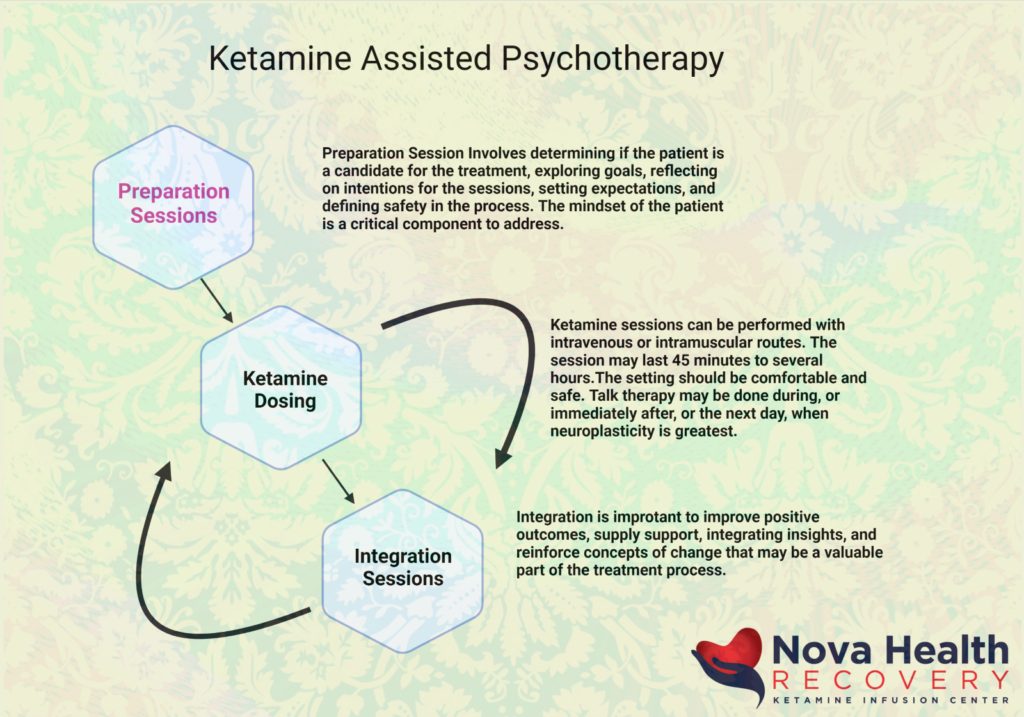
- Ketamine-assisted psychotherapy (KAP), combining doses with therapy for deeper integration.
- Telehealth platforms mailing oral lozenges with virtual support.
Safety concerns persist—high costs, variable protocols, and potential overuse have prompted calls for more oversight. Unlike cannabis dispensaries, ketamine operations are medical facilities, not retail, with stricter healthcare compliance (e.g., HIPAA, anti-kickback laws).
Security Implications for Providers
For companies offering security solutions to cannabis businesses, ketamine clinics present a parallel opportunity:
- Controlled Substance Handling — Clinics must follow DEA rules for secure storage (locked cabinets/safes), inventory audits, video surveillance, and access controls—similar to cannabis seed-to-sale tracking but under medical facility standards.
- Facility Risks — Rising patient volumes and valuable drug stocks make clinics targets for theft/diversion. Telehealth models add cybersecurity needs for patient data and prescribing records.
- Compliance Overlap — Many requirements mirror cannabis (background checks, alarm systems), but with added medical board audits and malpractice risks.
As psychedelic reforms advance slowly, ketamine remains the "here and now" option, filling gaps in mental health care. A blog post on this could highlight trends, regulations, and why clinics need robust security—positioning your services as essential in this expanding space.


Ketamine vs. Psilocybin Therapy: A 2025 Comparison
As of late 2025, both ketamine-assisted therapy and psilocybin-assisted therapy represent breakthrough options for mental health treatment, particularly treatment-resistant depression (TRD), anxiety, PTSD, and addiction. Ketamine is widely accessible in clinics, while psilocybin remains limited to regulated programs in a few states (e.g., Oregon, Colorado) or clinical trials. Here's a head-to-head comparison based on mechanisms, efficacy, experience, accessibility, and safety.
Key Differences at a Glance
| Aspect | Ketamine Therapy | Psilocybin Therapy |
|---|---|---|
| Mechanism | NMDA receptor antagonist; boosts glutamate, enhances neuroplasticity via mTOR pathway. | 5-HT2A serotonin receptor agonist; promotes profound introspection and default mode network reset. |
| Onset of Effects | Rapid: Hours to days after infusion. | Rapid onset during session, but peak therapeutic effects in days to weeks. |
| Duration of Benefits | Short-term: Weeks to months; often requires repeat sessions (e.g., 6 infusions initially, then maintenance). | Longer-lasting: Up to 6–12 months after 1–2 sessions in trials. |
| Typical Session | 40–60 min IV infusion or nasal spray; dissociative, detached feeling. | 6–8 hour guided session; intense emotional/mystical experience. |
| Number of Doses | Multiple (series of 6+, boosters). | Few (1–2 high doses with preparation/integration). |
| Subjective Experience Contribution | Low (5–10% of benefits tied to dissociation). | Higher (up to 24% linked to mystical/peak experience). |
| Efficacy in TRD | Strong rapid relief; 70–85% response rate initially, but fades without maintenance. Comparable to psilocybin in short-term trials. | Promising sustained remission; effects persist longer in many studies. |
| Accessibility (US) | Widely available in clinics (off-label IV/oral; FDA-approved esketamine nasal spray). | Limited to licensed service centers in OR/CO or trials; investigational elsewhere. |
| Safety Profile | Generally safe in clinical settings; risks: elevated BP, dissociation, potential abuse. No major cognitive decline. | Safe under supervision; risks: temporary anxiety/"bad trip," BP changes. Low abuse potential. |
Efficacy and Clinical Evidence
- Ketamine: Excels in rapid antidepressant effects, often within hours—ideal for acute suicidality or severe TRD. Long-term studies show sustained benefits with repeated dosing, but effects can wane. Esketamine (Spravato) is FDA-approved for TRD.
- Psilocybin: Often produces more durable changes with fewer sessions, linked to profound psychological insights. Trials show reductions in depression scores lasting months to a year. Animal models and human follow-ups suggest persistence beyond ketamine.
- Head-to-head: Network meta-analyses rank both highly for TRD efficacy and tolerability, often alongside ECT. Psilocybin may edge out in longevity, while ketamine wins on speed and availability.
Which Might Be Better for You?
- Choose ketamine for quick relief, easier access, or if multiple sessions are feasible.
- Consider psilocybin (where available) for deeper, longer-term transformation, especially if seeking existential or emotional breakthroughs.
Both are administered with psychotherapy for best outcomes—preparation, dosing, and integration are key. Research evolves rapidly; consult a licensed provider for personalized advice, as individual responses vary. If you're exploring options, start with what's legally accessible in your area!











